Penicillin’s successes trigger a major search for microorganisms with antibacterial action. Are there any more productive penicillin producing mould varieties out there? Yes, there are. The Italian pharmacologist Giuseppe Brotzu discovers the mould Cephalosporium Acremonium in a sewage outfall at the coast of Sardinia, in 1948. The raw filtrate of the mould appears to be able to master the infamous Staphyllococcus Aureus. It contains cephalosporins, a natural variation on the penicillin motive. This leads to a large number of successful antibiotics with many applications, down to the present time.
Project ‘100 years of antibiotics’
Episode 28. The success of Bacinol
Episode 29. The Gist factory, from illegal to global player
Episode 30. Cephalosporins
Episode 31. Help from an unexpected source
Episode 32. Turning point 1950

Lessons learned
Edward Abraham, a member of the well-known penicillin research group in Oxford, starts right away researching the new mould. But again, success takes its time. Ten years later, when on a skiing holiday, the correct structural formula dawns on him; and once more, the famous Dorothy Hodgkin confirms his view. Abraham slightly alters the cephalosporin molecule and in so doing, develops a very useful medicine. Having learned the lessons from the past, Abraham now does file for a patent. Eli Lilly, an American firm, starts marketing the first successful cephalosporin, cephalotin, in 1964. The royalties keep coming to Abraham and he becomes a rich man. However, most proceeds go to three foundations that stimulate research in a broad context. At the time of writing, they command some 200 million pounds. Oxford University has received tens of millions in the course of time, among others for the construction of labs and of congress centres. And the foundations have granted many scholarships to students.

As shown in episode 23, Abraham and colleagues in the end arrive at three building blocks, 6-APA, 7-ACA and 7-ADCA. This results in a multitude of successful medicines: the semi-synthetic penicillins (SSPs) and cephalosporins (SSCs). At present, we are living through the fifth generation of cephalosporins.
Cephalosporins, mode of action
Almost always, SSCs and SSPs have the same mode of action. In popular terms: these substances punch a hole in the cell wall of the bacteria under attack. The wall leaks, the bacterium is emptied, cannot propagate and dies. We now know the mechanism of this process down to each molecular step. The primary battlefield lies outside the bacterium. A growing bacterium (characteristically present in a new infection) needs to extend its cell wall continuously. The bacterium does this by transporting the construction materials from the inside of the cell to the exterior, through the growing cell wall, and assembling them there to new wall parts. One of the enzymes that couple those two important elements, also has a good binding location for an SSC or SSP at the very same spot where construction takes place. But once in place, the SSC or SSP cannot be removed anymore and hence, construction stops. The secret weapon is the beta-lactam bond in the SSC/P. This is opened up by part of the aforementioned enzyme, and the loose ends bind to the enzyme on both sides.
Resistance
The most obvious pathway for the bacterium to disarm the antibiotic is of course to defuse its secret weapon. Although generally speaking, there are other options. For instance the bacterium could just slightly alter its cell wall, in order to hide the point of attack for the antibiotic. And there are mechanisms consisting of pumping out the intruders as fast as possible.
Resistance to penicillins and cephalosporins almost invariably rests on the activity of a so-called beta-lactamase enzyme; an enzyme that cuts the crucial beta-lactam bond. By trial and error (and a large number of mutations), most pathogenic bacteria have developed such an enzyme. And the resistant bacterium takes good care to release the parts cut loose immediately, allowing it to attack the next molecule. The smartest bacteria start sending their lactamases through the cell wall as soon as an SSC of SSP comes near.
A never ending struggle
So far, our story might have led readers to the conclusion that we gloriously won the 100 year battle between chemistry and bacteria. But from now on we will highlight again and again that there is no winner in sight. Right now, a gloomy mood prevails; once more the possibility of lethal infections scares us. And yet, those threats do not by far compare to the level that our (great)grandparents had to face. We have a large number of medicines to our disposal, and most patients did not (yet) come across resistant bacteria. But expectations for year 2050 are that millions of people may then die from resistance, and that makes us pretty nervous.
We developed new variants of existing medicines again and again, and that has put us at an advantage for a long time. We will describe the smartest examples of those discoveries. They all rely on cheating the lactamase enzyme, used by the bacterium to fend off its attacker. One of these mechanisms involves the combination of two antibiotics; for instance an SSC or SSP and erythromycin. The resistant bacterium will attack the SSC or P and defuse it; but then, the erythromycin has already made its way inside in order to do its job. Other procedures use two different beta-lactam products, of which the bacterium clearly prefers one of both.
Clavulanic acid
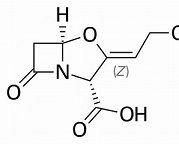
The most successful example involves the combination of the antibiotic with clavulanic acid. This procedure has extended the usefulness of the most successful SSP, amoxicillin, with several decades. A success that is still going on. The combination with clavulanic acid leads astray the bacterium’s lactamase. Clavulanic acid doesn’t have any antibiotic properties of its own, but it does contain the beta-lactam bond, highly preferred by the resistant bacterium. And then, amoxicillin can do its job as it used to do. Augmentin is one brand names for this combination. This has been discovered by the British company Beecham, in the ‘70s and ‘80s of the past century. But clavulanic acid is not the product of clever chemical research; it is produced by a strain of streptomycin and therefore seems to be part of the weaponry used by microorganisms in their battles. Clavulanic acid is active too when combined with other SSCs or SSPs, but the combination with Amox is by far the most popular.
This combination is also in use on the veterinarian market. It is being administered to dogs, cats, cattle and pigs. In combination with prednisone, the compound is used for drying off in-calf cows, and controlling udder infections. But more and more, use in animal fodder is either controversial or being banned. For in order to retain Augmentin’s high efficiency as a medicine for humans, the veterinary sector will have to start looking for alternatives.
Sources:
Wikipedia: all names and products mentioned
The Mould in Dr Florey’s Coat. Eric Lax, Abacus 2004. ISBN 978-0349-11768-3
Antibiotics: from prehistory to the present day, Kate Gould, Journal of Antimicrobial Chemotherapy 71, 572-575(2016)
N0-nonsense Guide to Antibiotics, Moira Dolan, SmartMedInfo, 2015; ISBN: 978-09968860-2-4
History and Current Use of Antimicrobial Drugs in Veterinary Medicine, John F Prescott, Microbiol. Spectr. 2017
