In a recent series on the invaluable site The conversation, Australian scientists raised the question to what extent we will in the future be able to rely on antibiotics. Resistance to antibiotics is on the rise, all over the world. The UN project that drug-resistant diseases could cause 10 million deaths a year by 2050. If antibiotics no longer protect us from illnesses, what can we do?
On this site, we already devoted an entire series on development and limitations of antibiotics. What do the experts themselves say? Well, their judgment is not far removed from ours. Let’s have a look.
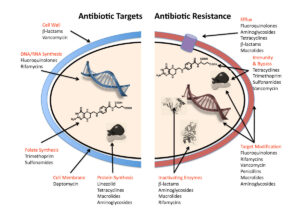
Alas, resistance to antibiotics will always develop
The problem with the failing activity of antibiotics has a name: resistance. ‘Resistance arises when bacteria are exposed to levels of antibiotics that don’t immediately kill them,’ Mark Blaskovich writes. If they’re not killed, they will develop defences against this antibiotic. More in general, there is always a minute fraction of bacteria that will survive the treatment. So, in the end, resistance will always develop – although much faster so if the medicine is being administered too shortly, or in an insufficient quantity.
Bacteria can resist the action of an antibiotic through a number of ways. They can make thicker cell walls, preventing the substance to enter. They can pump out the antibiotic as soon as it has entered the organism. They can alter the antibiotic’s target and then become invulnerable to this specific compound. Or they can modify the substance, rendering it harmless. But Blaskovich ends on a positive note: bacteria are ‘not yet’ invincible.
Overprescription
Much resistance arises from incompetent or inappropriate administration of drugs, writes Allen Cheng. Antibiotics are often continued for several days unnecessarily. That is because we prefer to err on the safe side. And many patients require the doctor to cure them, which will cause the use of stronger medication than strictly needed. And then, patients may become allergic to penicillins; or claim to be so. But often, write Winnie Tong and Jacqueline Loprete, tests show that this is not the case.
A major problem is that our inventory of antibiotics isn’t filled quickly enough in order to make up for antibiotics that have become ineffective because of resistance. Often at an early stage. In fact, more than 98.5% of newly discovered antibiotics never make it out of the lab. ‘The field of antibiotic discovery is littered with drugs that have failed to progress beyond the early stages of research,’ Sacha Pidot writes. But always, there is hope. In this case, from a substance called clovibactin. It has a different mode of operation. It doesn’t break down the cell wall, allowing our immune system to finish the job. Instead, it targets bacteria from the inside out. But it hasn’t been approved for general use yet.
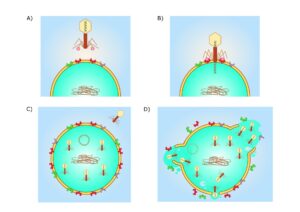
Phages
Another option is to revert to phage therapy, as described by Christine Carson and Lucy Furfaro. Phages are minute creatures that can infect bacteria and render them harmless. But phages are very specific in their actions. They will just infect one bacterium and leave others untouched. And it may take quite some time to find the correct phage for a bacterium. But then, even if we do so in a number of days, it needs to be manufactured and administered to the patient. Therefore, using phages to cure patients may be very cumbersome. ‘The customisation involved in delivering phage therapy takes a lot of time, labour and resources. This could make phage therapy relatively expensive,’ the authors write. Nevertheless, the treatment may be very effective.
Antibiotic resistance may be particularly high in developing countries, writes Gemma Ware. She cites Nubwa Medugu, a clinical microbiologist in Nigeria who tells her that in Nigerian hospitals, it is now it’s now ‘almost impossible’ to find infections that are not resistant to at least one antibiotic. That the number of infections resistant even to antibiotics of last resort is growing, is most worrying.
Do not overdose
But do we always need to take antibiotics? Many patients tend to believe that these are miracle drugs, and therefore ask for them. Or alternatively, GPs prescribe them because they feel patients require them. They play an important role. But GPs, write Mina Bakhit and Paul Glasziou, need to restrict themselves. This includes the use of decision guides to decide if antibiotics are really necessary, supplying patients with information on when antibiotics aren’t needed, and giving a ‘delayed prescription’ – only to be used after the patient waits to see if they get better on their own. Also, GPs need to get feedback on their prescription strategies. Sweden proves that the use of antibiotics can be halved – since the late 1990s, doctors were informed in a national restriction scheme.
What aggravates the problem, writes Christine Carson, is that comparable problems occur with other infectious organisms: fungi, viruses and parasites. True, the conditions that they cause, have a lower incidence than bacterial infections. Nevertheless, these can be serious. Like invasive fungal bloodstream infections or candidiasis, an infection of the vulva. And the number of antifungal medicines at our disposal is much lower. There are even less antiviral agents. Just two for instance, in the treatment of covid. Comparable in the case of anti-parasitic drugs.
The problem of resistance
The evolutionary process that leads to the emergence of resistance is inevitable, writes Carson. But still, we can do some things to minimise this. Using less antimicrobial agents. We need to reserve them for when they are really necessary; rather than using them ‘just in case’. They are precious resources. We need to preserve their effectiveness and not waste it. Cautious use is imperative. This will lend us time. Maybe, we will then discover more medicines and treatments that will treat patients. Even though at the moment, this is mainly speculation.
Interesting? Then also read:
Resistance to antibiotics
Chemistry vs. antibiotics. Phage therapy, a promising alternative to antibiotics?
New antibiotics – their development stagnates. Why?
