Just a short while ago, people hardly knew about viruses as a medicine. In particular, this is true for bacteriophages (or phages, for short) – viruses that eat bacteria. Until less than a century ago, we even didn’t know about the existence of such phages. Because they eat bacteria, phages can be used as a medicine. As an alternative to, or as an addition to antibiotics. But because of the early successes of antibiotics, they weren’t taken seriously for a long time. Because of mounting resistance against antibiotics, there is a renewed interest now in bacteriophages. And now there is a survey book (in Dutch).
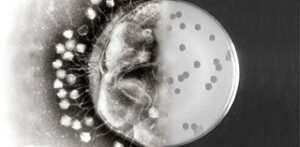
A never ending competition
For many centuries, phages have been the adversaries of bacteria. Probably for two billion years already. But phages are very specific. Each bacterium has a phage (or phages) of its own. The majority of phages are lytic phages. These kill almost instantly the sensitive bacterium. The phage injects it’s genetic material into the bacterium. This induces the bacterium to produce many more phages. Then, the cell wall breaks open and the phages are set free. These immediately infect new bacteria. But, and this is the point, just the bacteria affected by this specific phage.
Phages recognize a receptor on the bacterium. Receptors are very specific and even not identical in all otherwise similar bacteria. Therefore, when controlling a bacterium, we need to find exactly the phage that recognizes the bacterium’s receptor. Contrary to an antibiotic cure, a phage cure is very individually targeted. Moreover, bacteriophages moving into a bacterium may entice mutations in the bacterium, causing resistance to this specific phage. A mechanism that we often run into, in phage treatments.
Resistance against bacteriophages
It sometimes happens that bacteria become resistant to a certain phage within a few hours. But here’s a fortunate condition: phages too adapt miraculously fast. In short, phages and bacteria are in a mutual evolutionary process for as long as they exist – we cannot evade it. Because of the entire process of attacking the bacterium involved, resistance development, and adaptation of the phage to the new situation, bacteria are often fought with phage cocktails. Either cocktails with a number of phages attacking the same bacterium, or cocktails that attack a number of bacteria. But the upshot of this is that we incessantly need to adapt our fight against bacteria – just like phages adapt to our chemicals. The philosopher’s stone in this process would be the discovery of a mechanism used by bacteria to develop resistance – but so far, this has proven to be a far-fetched goal.
One method of circumventing resistance development is bacteria would be the simultaneous administration of antibiotics and phages. Or lending a broader activity to phages, allowing them to circumvent the defense mechanism of the bacterium. In another method, we could administer phages one by one. Say the phages leave alive just 1% of the bacteria, the subsequent administration of other phages could make a big difference. But also here, the final result of the battle between bacterium and bacteriophage will not be decisive – nature will always find a pathway behind the back of the phage. Although the bacterium might be weakened by this battle, which could give room to antibiotics to end the fight to their advantage.
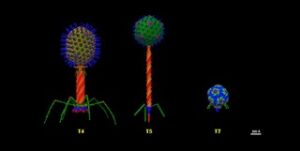
Specific treatments
For a long time, bacteriophages were only studied from behind the ‘iron curtain’, the dividing wall erected in Europe between NATO and Warsaw Pact countries. In the Western world, phages had almost become unknown. Their knowledge had gone to Georgia, in a corner of the Soviet Union; whereas in the West, antibiotics had become the medicines of choice.
But, as indicated before, antibiotics appeared not to be the miracle agents they were supposed to be. Resistance against them was mounting, sometimes shortly after their introduction. In the end, this led to a renewed interest in phages. Even though these carry problems of their own. For contrary to antibiotics, phages are very specific. There are no ‘broad spectrum’ phages. We need to search the appropriate phage to any infection. So, although Van den Brink devotes an entire chapter to ‘spectacular examples of successful treatments with bacteriophages’, each time it is exciting to see whether the correct phage will be found.
Everywhere, but quite specific
Phages exist everywhere next to bacteria. By the end of the nineteenth century, researchers discovered that bacteria can be killed – they just didn’t know what killed them. Twenty years later, D’Hérelle discovered that ‘bacteriophages’ cleaned up the bacteria. And then it lasted another twenty years for researchers to see actual phages, using an electronic microscope.
Bacteriophages are very specific, which renders them difficult in practice. Firstly, they need to be discovered. But precisely because of that specific character, they are very effective. A bacterium invaded by phages will burst after some twenty minutes, a process that will release tens to hundreds of new phages. Within several hours, this process will produce an enormous amount of phages, and the bacterium will kick the bucket. The good thing about this is that resistance to antibiotics has nothing to do with resistance to phages. Therefore, even ‘difficult’ infections can still be countered by phages – provided that one can find the correct phage.
The need for a flexible guideline
But there is a problem. Antibiotics nor phages will kill all toxic bacteria. They may do so in combination. But there is just a limited number of conditions for which we have the correct phage at hand. Moreover, we don’t know for how long phages will remain active. We don’t even know the best way of administering bacteriophages to a patient.
Moreover, existing guidelines for admission of phage products aren’t tailored to phages. The European Medicines Agency EMA has drawn up a guideline to which bacteriophages will have to conform in order to be admitted for use. But so far, no product conforms to that guideline. Moreover, bacteria often change their makeup under the influence of a phage, rendering resistance to them. That requires the phage to change itself. It does so fairly quickly – but then, changing the guideline with the same speed isn’t possible. Consequently, we need a flexible solution that can be implemented quickly; but so far, that bureaucratic puzzle hasn’t been solved. Perhaps, mounting antibiotics resistance might incite attempts to find a solution to this problem.
Doctor’s toolbox
Fortunately, over the past few years successful phage treatments have been published. So now, these come out of the dark. Financing them might still be a problem – each European state has different solutions to that issue. And yet, there is an urgent need for such solutions. Resistance to antibiotics rises much faster than the development of new therapies. Therefore, as far as we can judge now, bacteriophages will secure a place in the doctor’s toolkit. For instance, they work very well against infections caused by artificial materials like artificial hips. On them, often biofilms are formed; after a while, these cannot be counteracted with antibiotics. Phages would be able to support the antibiotics, by breaking down the biofilm.
But, even though phage treatments are often successful, it also happens that such a treatment doesn’t have success. Often, it is unpredictable in which patient the treatment will not succeed. Therefore, some researchers make a case for an international data bank that would store all experiences with phages.
Lysines
Lysines are ‘an improved version of the enzymes produced by bacteriophages that break open bacteria and kill them. Because of their very specific mode of action, lysines will only kill the bacterium towards which they are directed, and leave the rest of the flora untouched.’ There are synthetic lysines also, potentially ideal antibacterial precision agents, that can be produced against ‘in principle almost all bacteria’ (p.237).
By using lysines, we could reduce the use of broad spectrum antibiotics; agents of which we now discover a mounting evidence of disadvantages. They disrupt the balance in our intestines and are being linked to conditions like Parkinson, Alzheimer and Multiple Sclerosis. Lysines are more compatible with the microbiome. Lysines and bacteriophages are complementary to each other in a sense. Lysines are more suited for treatment of acute infections, phages for treatment of chronic infections. Therefore, lysines are a perfect complement to phages. Even though lysines themselves also need to be approved as medicines.
Towards precision medical science
Treatment of bacterial infections will increasingly become a matter of precise interventions. Medicines will become directed very specifically towards that single bacterium. Precision antibiotics like lysines will make a big difference to treatments. We might even very precisely target ‘difficult’ bacteria by adapted lysines. Also phage therapy, not yet exactly grown-up, may introduce revolutionary change.
Van den Brink concludes his book by the remark that bacteriophages will most likely ‘play an ever more important role in the future’ (p.256). But he immediately also issues a warning. Rules and guidelines still lag behind discoveries about phages. Work is being done there. On international congresses, there is a mounting interest in phages. WHO as well has put the issue on the agenda. ‘Bacteriophages, so it seems, have started a now unstoppable advance.’
Interesting? Then also read:
Bacteriophages, we need them
Chemistry vs. bacteria. Bacteriophages, almost forgotten, but….
Chemistry vs. bacteria. Phage therapy, a promising alternative to antibiotics?
