Electricity should preferably be consumed immediately when it is generated. Storing electricity is not that easy. It always comes down to converting electricity into something else, that is used at a later date to generate electricity when needed. Talking about electricity, it is always about large quantities. So, flashlight batteries are irrelevant. Nevertheless, other-type batteries are the most obvious form of large-scale electricity storage. Storing electricity is important because sun and wind sometimes supply too much, and sometimes too little, in order to meet the demand for electricity.
Until the turn of the century, the lead battery was the only way to store electricity on such a large scale that it could be used to start a car engine. Around that time, lithium-ion battery technology emerged, a revolutionary development [1]. If fully charged, cars can travel several hundred kilometers on this battery.
But in addition to electric cars, batteries can also be used in an electricity network. This is referred to as grid or network batteries. Many consider this option to be too expensive, but it is questionable whether this is a correct assumption [2]. Below I explain why 2 cents/kWh in LFP battery could be possible.
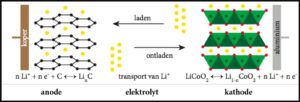
What does a battery look like on the inside?
A battery consists of cells that are built hundreds of times in a row. A cell is nothing more than two metal plates (electrodes) with a liquid (electrolyte) in between. One of the two electrodes is the anode, the other is the cathode.
Schematic representation of one compartment in a lithium-ion battery (https://www.betase.nl/images/Li-ion-batterij-principe.png) [3]. Legend: koper = copper, aluminium = aluminum, laden = charging, ontladen = discharging, elektrolyt = electrolyte, transport van Li+ = transport of Li+
In a lithium ion battery, the anode is made of copper, covered with a layer of graphite on top. This is not in the form of pure graphite, but graphite as the main component being packed in a kind of paint layer. During charging, electrons are pumped to the anode, where they convert lithium ions into lithium atoms. Subsequently, those lithium atoms are absorbed into the graphite.
- Anode (charging): Li+ + e– => Li
The cathode is made of aluminum with a similar layer on top, which contains cobalt oxide. This is often in the form of the lithium salt of nickel-manganese-cobalt oxide, hence the designation NMC battery. During charging, electrons are pumped away from the cathode. As a result, the cobalt ions change from the trivalent form (LiCoO2) to the quadrivalent (CoO2). This reaction releases lithium ions, which eventually end up on the anode.
- Cathode (charging): LiCo(III)O2 => Co(IV)O2 + Li+ + e–
During discharging, the reverse reactions take place.
In the electrolyte, the lithium ions need an anti-ion and that is hexafluorophosphate (PF6–). The solvent must be ‘aprotic’, i.e. free of protons (H+ ions). Because water molecules are created from H+ ions and they significantly shorten the lifespan of this battery. One of the very few options for lithium is lithium hexafluorophosphate (LiPF6) dissolved in methyl ethyl carbonate. A mixture of methyl and ethyl prevents this solvent from crystallizing at low temperature.
Alkyl carbonates are easy to make, in this case from methanol (CH3-OH), phosgene (Cl-(C=O)-Cl) and ethanol (C2H5-OH). Phosgene is the raw material for polyurethane, better known as PUR foam.
- CH3-OH + Cl-(C=O)-Cl + C2H5-OH => CH3-O-(C=O)-O-C2H5
This is different with LiPF6. Here are the chemical steps needed [4]:
- H3PO4 + 6 HF => HPF6 + 4 H2O
- LiCl + HPFl6 => LiPF6 + HCl
Although the world’s most lithium is mined outside of China in the form of LiCl, this country is the largest producer of LiPF6.
Then we come to graphite. This is a special form of carbon, which can be synthesized from aromatic hydrocarbons, but can also be extracted through mining.
Finally, the cobalt oxide. This is still the standard in electric cars, but in China, the LFP battery is starting to emerge. LFP stands for ‘lithium ferro phosphate’. This is a lithium-ion battery, in which cobalt oxide has been replaced by iron phosphate. Nickel and manganese will also no longer be needed. All that makes this battery a lot cheaper.
- Cathode (charging): LiFe(II)PO4 => Fe(III)PO4 + Li+ + e–
But the developments go further: In China, the first electric cars are now driving around on sodium-ion batteries [5]. That is even cheaper.

How much does an alkali-ion battery cost?
So much for the chemistry of alkali-ion batteries. Other important aspects are ‘energy density’, ‘charging cycles’ and ’round-trip efficiency’.
First of all, energy density: A lithium-ion battery can store 0.25 kWh (kiloWatt-hour) per kg, while for a sodium-ion battery it is 0.16 kWh/kg. For an electric car, this means a significantly shorter range between charges. But for a network battery, a lower energy density is hardly a problem. In comparison, the energy density of gasoline is around 10 kWh/liter.
Then there are the ‘charging cycles’: By charging and discharging again and again, the energy density gradually decreases until it has become unacceptably low and the battery has to be replaced. In a network environment, a decrease in energy density of e.g. 20% after a year can easily be solved by adding 20% more batteries in the course of that year. This is not possible in an electric car.
Finally, round-trip efficiency: How many kWh do you get back for every kWh you put in? For alkali-ion batteries, this is above 90%. That is almost 3 times higher than for storage with green hydrogen, which is produced with an electrolyzer and then used to generate electricity in a power plant. That combination has a round-trip efficiency of only 35%.
At the moment, an LFP battery costs about € 150 per kWh of storage capacity and this battery can deliver 1,000 – 2,000 charging cycles. Without interest charges, that comes out to 10 ct/kWh. With one charge and discharge every day, this battery lasts more than 4 years. Many electric cars with a lithium-ion battery still appear to have almost the same range even after 4 years of intensive use. This is a strong indication that twice as many charging cycles are feasible and thus a halving of the price from 10 cents/kWh to 5 cents/kWh.
As described above, an LPF battery contains the elements copper, carbon, lithium, iron and aluminum. On average, these ingredients cost at most €/kg 3. At kWh/kg 0.25, you need 4 kg to store 1 kg of electricity. That costs € 12. The battery now costs €150. That means that (150-12=) €/kWh 138 remains for assembly. The larger the scale, the lower the assembly costs. This justifies the expectation that the purchase price could drop from €/kWh 150 to €/kWh 50 [6, 7].
With these two developments, the price of storage of 1 kWh could go down by a total factor of 6 from 10 cents/kWh to 1.67 cents/kWh. Rounded off and including interest rates, I then arrive at 2 cents/kWh.
One of the largest battery clusters in the world under construction in California in 2021, with a total of 2.5 GWh of storage capacity [8].
REFERENCES
[1] Wikipedia (24 November 2024) Lithium-ion battery (https://en.wikipedia.org/wiki/Lithium-ion_battery)
[2] Auke Hoekstra (24 June 2024): Batteries: how cheap can they get? (https://aukehoekstra.substack.com/p/batteries-how-cheap-can-they-get)
[3] Eddy Brinkman (31 July 2018) Electrochemistry behind rechargeable lithium ion batteries (https://www.betase.nl/electrochemistry-behind-rechargeable-lithium-ion-batteries/?lang=en)
[4] Wikipedia (24 November 2024) Lithium hexafluorphosphate (https://en.wikipedia.org/wiki/Lithium_hexafluorophosphate)
[5] Wikipedia (24 November 2024) Sodium-ion battery (https://en.wikipedia.org/wiki/Sodium-ion_battery)
[6] Billy Wu (3 January, 2024; podcast) Sodium ion batteries – The low-cost future of energy storage? (https://www.youtube.com/watch?v=O3jjJb-CcCU)
[7] Jens Peters et al. in Batteries 2019 (5) 10: Exploring the Economic Potential of Sodium-Ion Batteries (https://doi.org/10.3390/batteries5010010)
[8] Andy Colthorpe in Energy Storage News, November 12, 2021: Batteries at world’s largest solar-plus-storage project supplying California community energy group (https://www.energy-storage.news/batteries-at-worlds-largest-solar-plus-storage-project-supplying-california-community-energy-group/)
All websites were accessed on December 8, 2024.
