Photosynthesis is one of the most important biological processes on earth, if not the most important one: no life without photosynthesis! The Rubisco enzyme plays a key role in this process. But from the viewpoint of modern agricultural science, the photosynthesis process is far from perfect. So commentators devoted much hyperbolic praise on a publication in Science, January 4 this year, that reported a 40% ‘boost’ in biomass production after genetic modification of the tobacco plant. These exaggerated formulations annoyed me, as they showed so clearly the arrogance with which we humans treat nature.
Hans Tramper is professor emeritus in Bioprocess Technology at Wageningen University and reflects on the development of his subject in a series of essays. His pieces were published so far on 18 June, 30 June, 11 July, 22 July, 19 August , 10 September, 21 September, 30 September, 10 October, 31 October, 8 November, 2 December and 26 December 2018, and 21 April and 28 May 2019.
My first acquaintance with Rubisco
From February 1, 1975 to September 1, 1979, I was a temporary assistant professor in the organic chemistry research group, in what then was called Wageningen University of Practical Agricultural Sciences. Back then, such an appointment would include mainly research for the PhD and some educational tasks. The latter included assisting in practicals and in correcting exams, a few times a year. My research was new; as I started, there was little more than the title: Application of enzymes in organic syntheses. I soon discovered that the biochemistry research group would be a better environment for conducting my experiments, and therefore I spent most of my time there. The two buzz words there were Rubisco and Vasolastine. Both were quite unconnected with my own work, but as they were much discussed, I learned my bit about them. Rubisco catalyses the first step in the Calvin cycle of the photosynthesis and this was the subject of fundamental research in the biochemistry research group. Now, 40 years later, it is central to the Science article and in the spotlights now. During my first year at biochemistry, a small research into the effects of Vasolastine on request of a general practitioner, evolved into a twenty-years’ war between our renowned professor the late Cees Veeger, and Enzypharm, the producers of Vasolastine. I would just like to link here to the interesting and instructive synopsis (in Dutch) of this conflict that got out of hand.
‘Don’t get annoyed!’
I am a competitive person, I am a bad loser and I easily get annoyed if I cannot control events. The person who suffered most from these characteristics was I myself, but in the course of half a century I managed to detach myself largely from them. Early this year however, things got out of hand again. It started on the morning of Saturday January 5, when I read my newspaper de Volkskrant. First the strip, Sigmund, and then Bert Wagendorp’s column, my favourite. It was called Breakthrough that day, an absolutely magnificent piece. He reacted on a story by his colleague Maarten Keulemans, who reflected on the Science article. Wagendorp writes: ‘According to our scientific editor Maarten Keulemans, most plants suffer from a ‘defective photosynthesis’, but this flaw has now been corrected. Long live the human intelligence! Photosynthesis (the production of carbohydrates and oxygen from CO2 and water with light energy) is the chemical reaction to which we owe everything, including ourselves.’
So far, so good. In the framework of a column, this is state-of-the-art terminology. But when I continued to read Keulemans’s piece on the reverse side of the page, the terminology started to annoy me. And my annoyance grew as I ran across more articles referring to photosynthesis in terms like ‘evolutionary sloppiness’ and ‘one of evolution’s greatest mistakes’. And my annoyance reached its peak when I read: ‘Intelligent design has triumphed where evolution has mostly failed. Biologists have boosted the biomass of tobacco by around 40 per cent by compensating for a fundamental flaw in photosynthesis.’ But the author of the article, Michael le Page, redresses somewhat this idea of an ‘evolutionary mistake’. In the paragraph titled Evolution’s greatest mistakes he writes: ‘The enzyme that grabs hold of CO2 and adds it to a carbon chain often grabs hold of an oxygen molecule by mistake. This generates toxic molecules that plants have to expend energy to mop up (…). To be fair, it wasn’t a huge issue when photosynthesis first evolved, because there was little oxygen around. But as oxygen levels rose and CO2 levels declined over the aeons, it became a huge problem for plants. The grabbing of oxygen by mistake – called photorespiration – now happens so often it can reduce the efficiency of photosynthesis by as much as 50 per cent. A few plants have evolved a solution: they concentrate CO2 inside them to reduce the odds of oxygen being grabbed by mistake. But most of the plants we eat, including almost all vegetables and fruits, and key crops such as wheat, rice and soybeans, can’t do this. Biologists have been trying to find a fix for decades.’
‘Nature makes no mistakes’
I am not the only one who got annoyed by such rather arrogant phrases. Agricultural journalist Marc van der Sterren was clear about that in his reaction titled Gentech does not prevent hunger on the Opinion & Debate page of de Volkskrant. I cite a few passages. ‘Nature does not make mistakes. Repairing a ‘sloppy photosynthesis’ has its benefits for the economy, but it will not solve a food problem. […] Without any scruples or quotation marks Maarten Keulemans uses terms like ‘flaws’, ‘sloppy photosynthesis’ and ‘poisons’. […] But would that constitute a ‘flaw’? The tobacco plant is simply from nature, and nature never intended to produce as much food as possible for a single species. On the contrary, the success of the evolution existed in the production of the enormous range of species in flora and fauna.’
‘We produce enough to feed one and a half times present global population’
‘Producing an ever increasing amount of food to feed mankind is a fine goal,’ Marc van der Sterren continued, ‘but that does not change a bit in the causes of hunger: most hungry people simply don’t have enough money to buy their food, or the knowledge to produce it. Even though a staggering amount of 80 percent lives in the countryside. The good news is: the gene technological solution to repair nature’s ‘flaw’ will be free of patents, so poor farmers will also have the opportunity to make use of it. The million dollar question is: why don’t we approach them with our present knowledge already, for them not to suffer from hunger right now? The million dollar answer: the multinationals that control the food system, will not earn from that strategy.’ A story that is close to my heart and… my annoyance disappeared.
Photosynthesis is hyper complex
Wagendorp also cites Louise Fresco, Wageningen UR’s chairwoman now for some years. ‘ “In our research, photosynthesis is the holy grail,” the Wageningen professor in food technology (sic) Louise O. Fresco told this paper, answering the question about the ten billion mouths. “Time has come to start improving on the efficiency of that process, in order to boost agricultural productivity. The process is hyper complex, and therefore we have not succeeded yet in improving on it.” In the interview, she made a plea for ample funding. At the University Illinois, where the breakthrough took place, they were granted such an amount of funds. At first, the research proposal met with funding problems. These were solved when Bill and Melissa (sic) Gates, the world’s richest philanthropists, came up with 25 million dollars.’ I second the idea that photosynthesis is a very complex process – that was made clear to me forty years ago already; even though Mrs Fresco is a professor but not in food technology, and Mrs Gates is Melinda and not Melissa. But in my own terms I would like to stress again what I supported in the preceding paragraph: that agricultural productivity should indeed become better, but that above all the distribution of food should become much more fair.
Rubisco
For me, the quest for the holy grail in photosynthesis should stop at Rubisco. That’s where the problems are, that’s where we should look in order to raise productivity, from the perspective of modern agriculture. In November 2000, this enzyme was called molecule of the month, in 2016 The great RuBisCO and its amazing carbon fixation and in 2018 the most important protein on earth. According to the 2016 article, Rubisco kick-starts the Calvin cycle that processes atmospheric carbon dioxide through carbon fixation to substances useful to the cell, primarily glucose. Motor cycles used to have a pedal, the Kickstarter, that started the engine. More often than not, one would have to kick it a few times before the engine got going. In its present sense, kick-starting something is getting it started quickly. But in the case of Rubisco, that cannot be the true meaning. Rubisco has a very low activity, relatively speaking, and therefore I prefer the metaphor of the Kickstarter, as the Calvin Cycle needs to turn around six times, getting started six times as it were, in order to produce one single glucose molecule.
Rubisco is the crucial enzyme in the Calvin cycle, and therefore no plant cells and hence no life on earth without this enzyme. According to the 2018 article, this by itself is sufficient reason to call this enzyme ‘the most important protein on earth’. Moreover, it is the most abundant protein on the planet. That is because it is rather inefficient. According to the 2000 article, most enzymes process some thousand substrate molecules per second, whereas Rubisco can capture just three carbon dioxide molecules per second. The plant cells overcome this by the production of Rubisco in major quantities.
Rubisco is not just slow by comparison; it also is not very specific on carbon dioxide and oxygen. Oxygen molecules are quite similar to carbon dioxide molecules in shape and physical-chemical properties. Therefore quite easily, the active centre of the Rubisco molecule binds them. This will happen more often if more oxygen molecules are around. If this happens, it will lead to compounds like glycolate that the plant cell has to remove, at the expense of energy. The photosynthesis boost published in Science is about the more efficient processing of this compound, more about that in the last paragraph.
In all respects, Rubisco is a special enzyme. One of the oldest and largest, it hardly changed in the course of the evolution. We can only speculate on the causes of that. For instance, Marc van der Sterren wonders if those ‘toxins’ that the plant produces may have a biological function. He even suggests that those ‘toxins’ might feed moulds, bacteria and other organisms that cooperate in an intricate and diverse ecosystem. An intriguing thought.
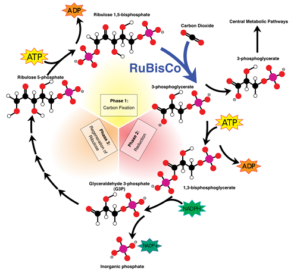
The Calvin cycle
In the plants chloroplasts Rubisco (ribulose-1,5-biphosphate-carboxylase-oxygenase) binds carbon dioxide to 1,5-biphosphate (RuBP), a sugar consisting of a string of five carbon atoms. The resulting C6 compound, bound to Rubisco, is then split in half by the enzyme. This produces two molecules of glycerate-3-phosphate (C3 compound). This compound can be used in several central metabolic pathways, but the bulk of it will be processed to glyceraldehyde-3-phosphate (G3P), generally looked upon as the most important product of the Calvin cycle. In order to arrive at that result, the chloroplast uses the energy contained in ATP and the reducing power of NADPH. It will then transport the side products ADP, inorganic phosphate ion and NADP+ to the light dependent part of the chloroplast, where light energy regenerates them to ATP and NADPH. Three times capturing a carbon dioxide molecule by Rubisco will produce six glyceraldehyde-3-phosphate molecules. Five of those will be recirculated, at the expense of more ATP, to the original compound ribulose-1,5-biphosphate in order to keep the cycle going. The sixth one can be directed right away into central metabolic pathways; alternatively, two molecules could be transformed into C6 sugars, primarily glucose. So, in order to produce one molecule of glucose, Rubisco will have to capture six carbon dioxide molecules. Glucose can be transported to other cells and undergo glycolysis there, for instance, or be stored in the chloroplasts themselves in the form of starch.
Disregarding water and oxygen, the reaction scheme can be represented as follows:
6 CO2 + 6 RuBP (+ energy of 12 ATP and 12 NADPH) → 12 G3P
10 G3P (+ energy of 6 ATP) → 6 RuBP
2 G3P → glucose (C6H12O6)
Overall, including water consumption and oxygen production, this boils down to:
6H2O + 6CO2 → C6H12O6 + 6O2
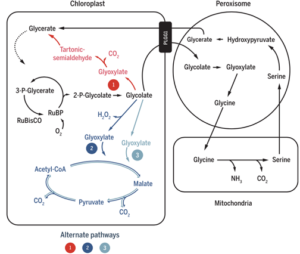
Photosynthesis boost
The vast majority of land-based plants, some 95%, are C3 plants. They are called by that name because their vitality is based on the C3 compounds of the Calvin cycle. The vast majority of the vegetal products in our food has been produced by C3 plants. All these plants show photorespiration to some degree, producing ‘toxic’ glycolate. This is being broken down in the peroxisomes and mitochondria, at the expense of energy (Figure 2). This can reduce the efficiency of photosynthesis by 20-50%. There are other developments that have a certain potential to boost efficiency, but here we just discuss the breakthrough technology published in Science early this year. The researchers tested the three most promising alternative photorespiratory pathways in field trials with a transgenic tobacco plant. Previously, they experimented for many years with many more alternatives in the lab and in the greenhouse. They chose the tobacco plant because this is an ideal model system. It is relatively easy to genetically modify the tobacco plant. Moreover, its life cycle is short, it has a rich foliage comparable to other crops, and produces many seeds. In Alternative 1, the researchers modified the tobacco plant with five genes from the glycolate oxidation pathway of an Escherichia coli bacterium; in Alternative 2, they did so with a glycolate oxidase of Arabidopsis, a malate synthase of a pumpkin and a catalase from E. coli; and in Alternative 3 with a pumpkin malate synthase and a glycolate dehydrogenase from a green alga.
And then, they didn’t just test these three alternatives, but from each alternative they also tested a version in which the chloroplast’s (PLGG1) own glycolate transport system had been disabled by RNAi; also see Part 4.4 and Part 4.7. The results from two growing seasons were clear. Alternative 3 performs the best. Without disabling PLGG1, yield increases by >25%, and with disabling even by >40%. I conclude by citing just one passage from Wagendorp, comparing it to the conclusion of the authors of the Science article. ‘The breakthrough resulted from the successful genetic modification of the tobacco plant,’ Wagendorp wrote. ‘And that’s a pity, in a time when tobacco has become much less popular, but the principle will most probably also work in other plants.’ And compare Science: ‘Engineering more efficient photorespiratory pathways into tobacco while inhibiting the native pathway markedly increased both photosynthetic efficiency and vegetative biomass. We are optimistic that similar gains may be achieved and translated into increased yield in C3 grain crops because photorespiration is common to all C3 plants […]’. ‘Optimistic’ sounds just a bit more cautious than ‘most probably’.
Interesting? Then also read:
Respectful treatment of the complexity of biomass
Cold recovery of potato proteins, a wonderful innovation
Nature as an inventor
